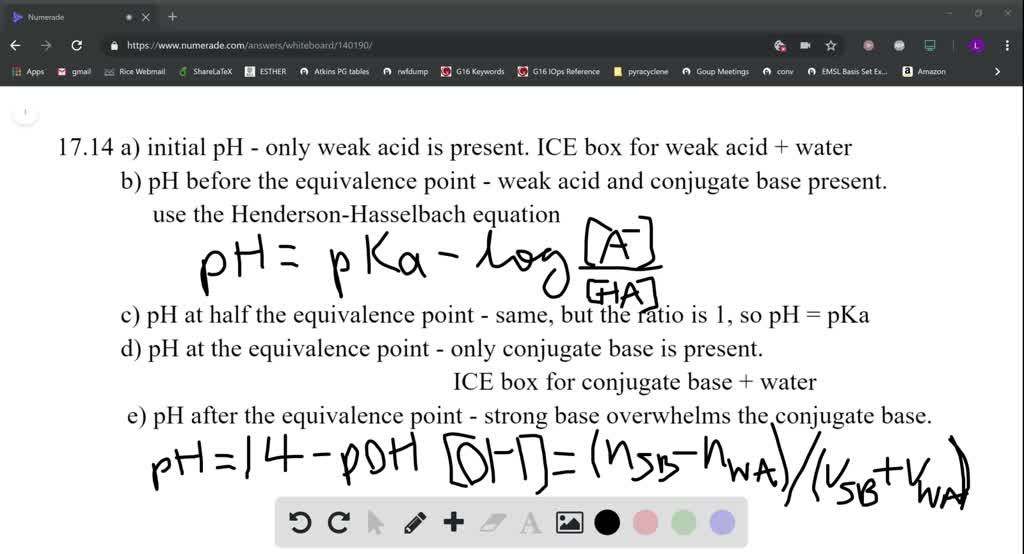
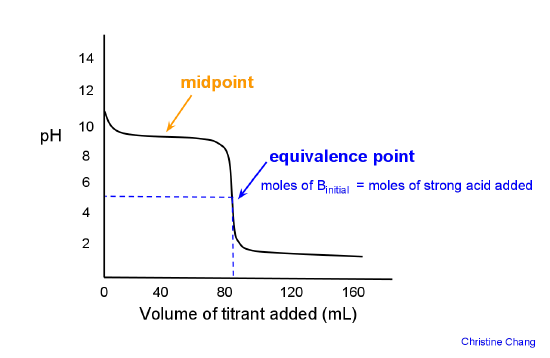
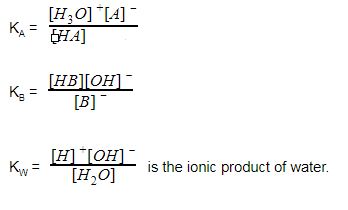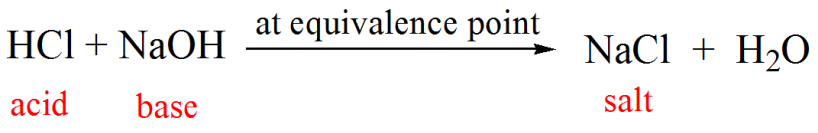![Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)] Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)]](https://d10lpgp6xz60nq.cloudfront.net/ss/web/491584.jpg)
Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)]

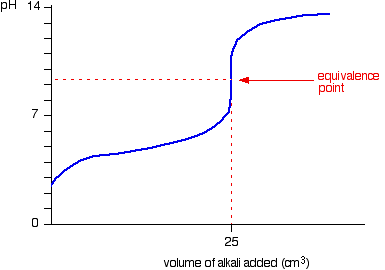
How to Calculate Analyte Concentration Using the Equivalence Point in an Acid-base Titration | Chemistry | Study.com
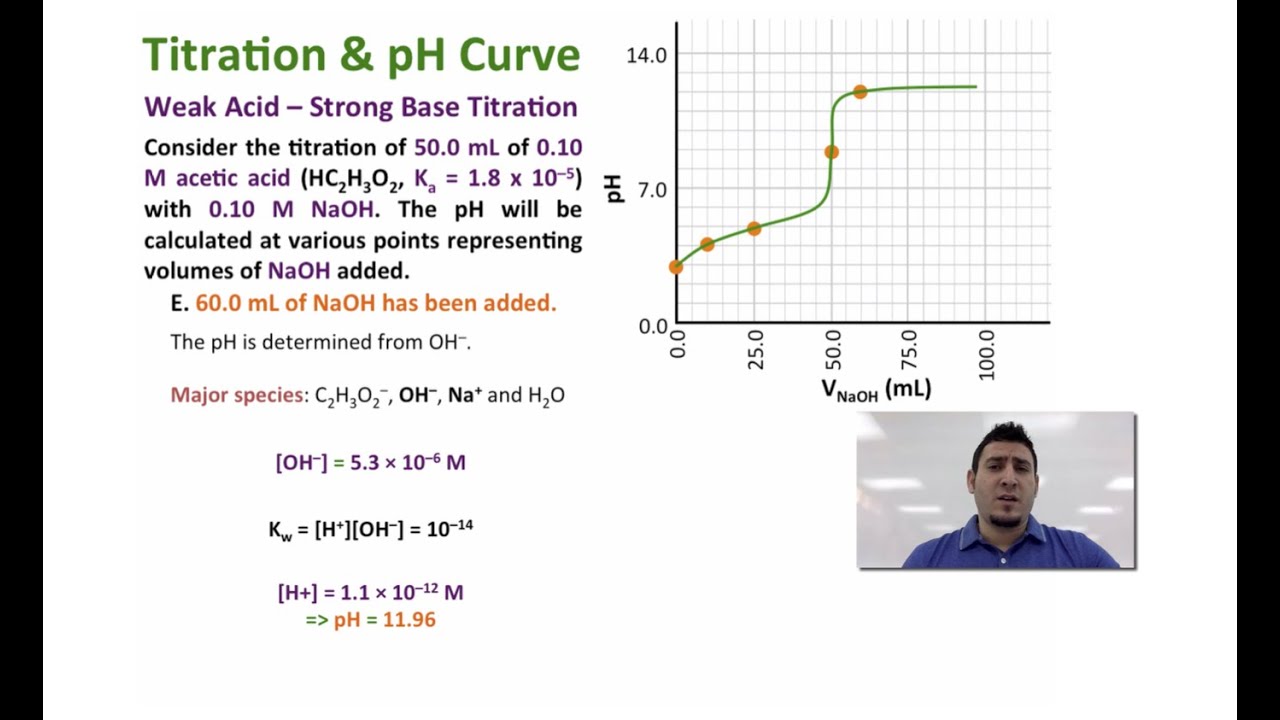
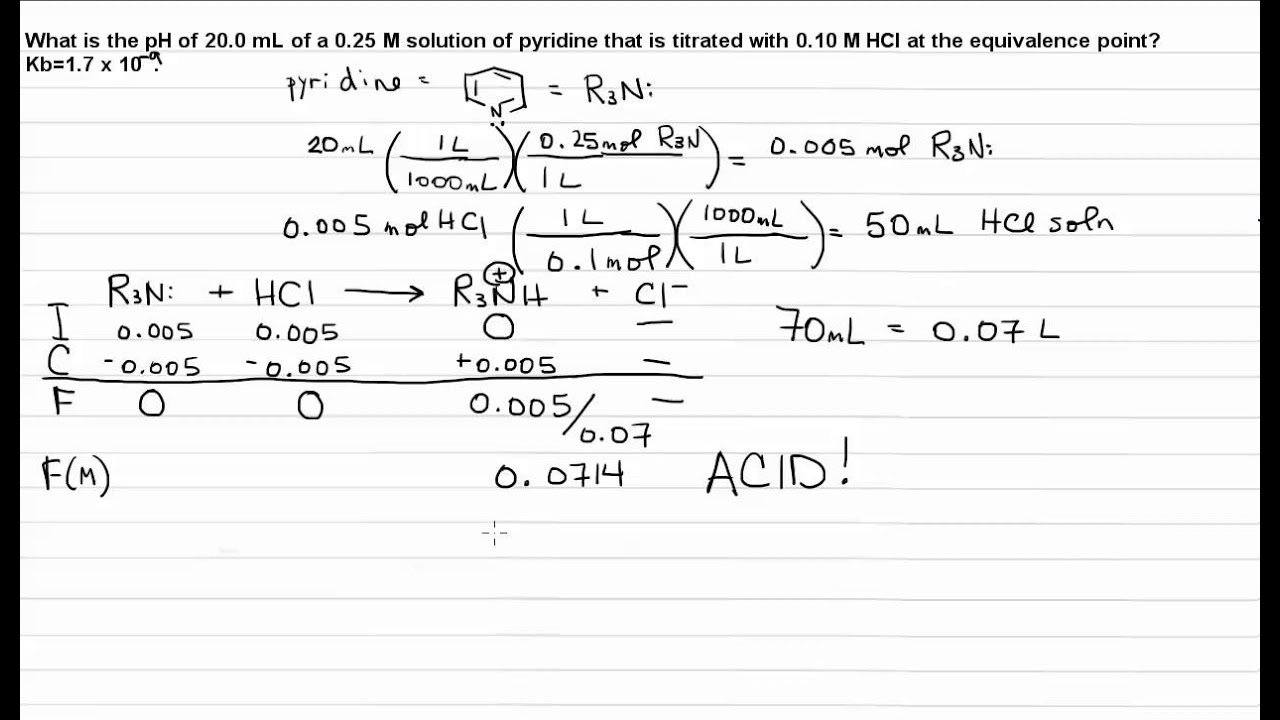
How do you calculate the pH at the equivalence point for the titration of .190M methylamine with .190M HCl? The Kb of methylamine is 5.0x10^-4. | Socratic

OneClass: Calculate the pH at the equivalence point for the following titration: 0.20 M HCl versus 0....
Calculate the pH at the equivalence point of the titration between 0.1M CH3COOH ( 25 ml) with 0.05 M NaOH. - Sarthaks eConnect | Largest Online Education Community

Calculate the pH at equivalence point of the titration between 0.1 M CH3COOH (25 ml) with 0.05 M NaOH Ka for CH3COOH = 1.8 × 10^-5 .
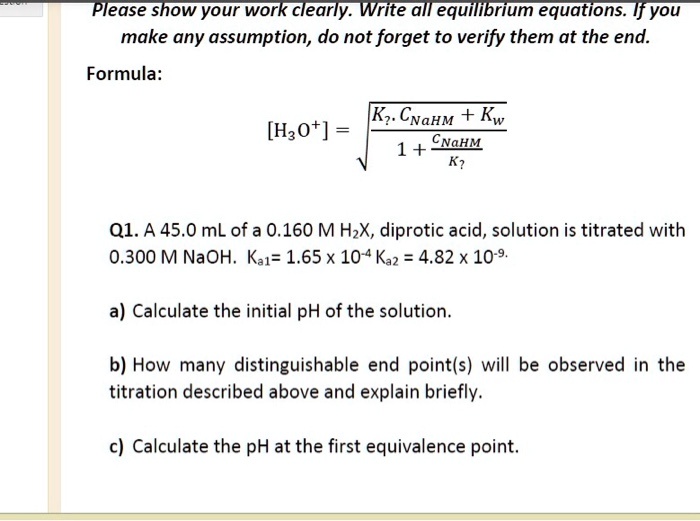
SOLVED: Please show your work clearly. Write all equillbrium equations. If you make any assumption, do not forget to verify them at the end: Formula: Kz CNaHM + Kw 1 + CNahm [
![Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)] Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/328699097_web.png)
Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)]